
VetStat Electrolyte and Blood Gas Analyzer
Run the tests you need most to support your patients—whether healthy or sick, young or senior, routine or critical.
- The only in-house blood gas analyzer modified specifically for veterinarians
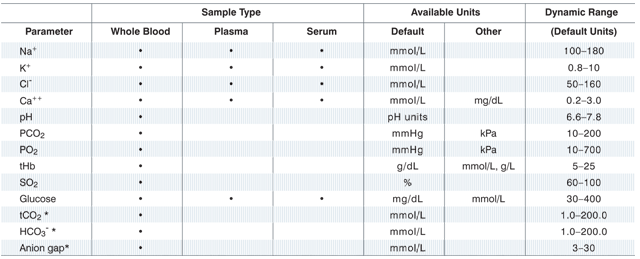
- Fast, results for electrolytes, acid-base status, blood gases, ionized calcium and glucose
- Runs whole blood, plasma and serum on single-use cassettes
- Designed for simple and rapid operation by any staff member
The VetStat analyzer connects to the IDEXX VetLab Station, a laboratory information management system that collects all results into an electronic medical record and prints them on an integrated report for easy interpretation.
Benefits
Comprehensive information for more informed and confident decisions
Easy to use
- User-friendly touch screen with simple menu-driven navigation
- Automated sample aspiration reduces staff time and training
- Whole blood, plasma or serum samples for electrolytes, glucose and ionized calcium results
- Whole blood samples for acid-base and respiratory results
- Room-temperature cassette storage so tests are ready to run at any time
Accurate
- On-board, species-specific reference ranges (canine, feline, equine, other)
- Each disposable, single-use cassette has its calibration curve information captured in its bar code—no data entry required
- Automated sample aspiration minimizes errors
Cost effective
- Single-use cassettes allow for a consistently low per-test price
- 12-cassette packaging significantly reduces the risk of expired product
Profitable
- Battery or AC-operated
- Rechargeable battery allows independent operation for up to 4 hours
Support
- Count on timely, responsive access to excellent customer service, technical assistance and educational support
Technology
Principles of Operation
The VetStat analyzer is a touch-screen driven instrument that measures optical fluorescence from discrete sensors called optical electrodes (optodes).
- A disposable, single-use cassette contains all of the elements needed for calibration, sample measurement and waste containment. Specific calibration information from the cassette is read into the analyzer by scanning the cassette bar code with the bar-code reader. The cassette is then placed into the measurement chamber.
- The analyzer warms the cassette to 37.0° ±0.1°C (98.6° ±0.1°F), and performs a calibration verification by passing a precision calibration gas mixture across the optode sensors.
- The pH and electrolyte channels are calibrated with precision buffer solution contained in the cassette. When calibration is verified, the analyzer aspirates the blood sample into the cassette and across the optode sensors. After equilibrating with the blood sample, fluorescence emission is then measured.
- After a single measurement, the cassette containing the blood sample is removed from the analyzer and discarded. The analyzer contains no reagents, blood or waste.
Specifications
| Species | Canine, feline, equine |
| Sample Size | 125 µL |
| Sample Application | Syringe, capillary tube |
| Sample Types |
|
| Calibration | <90 seconds, calibrated before sample is introduced |
| Analysis Time | <2 minutes |
| Measurement Principle | Fluorescence, reflectance |
| Dimensions | 124 x 362 x 230 mm (4.7 x 14.2 x 9.1 in) |
Test Menu
Room-temperature storage cassettes are ready to use immediately, so you’re prepared for anything.
Single-Use Cassettes
Each lot of single-use cassettes is calibrated during the manufacturing process. Each cassette package is then labeled with a bar code containing this calibration information, as well as its lot number and expiration date.
You can choose from 5 single-use cassettes:
| Cassette | Tests |
| Electrolytes |
Na+, K+, Cl– |
| Electrolytes 8 Plus | Na+, K+, Cl–, pH, PCO2, tCO2, HCO3–, anion gap |
| Respiratory/Blood Gases | Na+, K+, Cl–, pH, PCO2, tCO2, HCO3–, anion gap, base excess, PO2, SO2, tHb |
| Ionized Calcium | Ca++ |
| Glucose | GLU |
The cassette’s bar code is read by scanning the package with the bar-code reader. The single-use cassette is then installed and an automatic calibration verification is performed using a precision gas mixture and the sample cassette’s internal storage buffer.
How To / Resources
The IDEXX VetStat Analyzer is easy to use!
Step 1
 From the Home screen, scan the sample cassette bar code.
From the Home screen, scan the sample cassette bar code.
Step 2
 Wipe both sides of the cassette with a lint-free wipe to remove moisture. Insert the cassette into the sample measurement chamber (SMC), and close the cover.
Wipe both sides of the cassette with a lint-free wipe to remove moisture. Insert the cassette into the sample measurement chamber (SMC), and close the cover.
Step 3
 Enter the patient information and then tap Finish. Note: Patient information can be entered before, during or after the sample analysis.
Enter the patient information and then tap Finish. Note: Patient information can be entered before, during or after the sample analysis.
Step 4

Mix the patient sample and expel any air bubbles. Attach it to the sample cassette and tap OK.
Step 5
 Read the results.
Read the results.
Step 6
 Remove and discard the sample cassette. Close the SMC cover.
Remove and discard the sample cassette. Close the SMC cover.
That's it!
Six easy steps and your patient's results are ready in less than 2 minutes!
Resources
Questions and answers
Fluid Therapy
The subcutaneous route can be used for maintenance needs in small dogs and cats, but it shouldn't be used for patients with acute, severe fluid losses (i.e., patients with evidence of circulating volume compromise). Likewise, this route is not recommended for extremely dehydrated or hypothermic animals that may have considerable peripheral vasoconstriction, which potentially could interfere with absorption of the fluid. Only isotonic crystalloid fluids containing lactate as a base precursor (e.g., lactated Ringer's solution) are recommended for subcutaneous administration. The low pH and high acetate content of some Plasmalyte products and Normosol R may contribute to pain on subcutaneous injection. Five percent dextrose in water typically is not recommended for subcutaneous use because temporary electrolyte imbalance potentially may occur as extracellular fluid equilibrates with the administered electrolyte-free fluid. Lastly, there is some concern that use of dextrose-containing crystalloids subcutaneously could predispose the animal to cellulitis if bacteria are introduced on injection.
Five percent dextrose typically is used to replace a water deficit, and not to provide calories. Except in very small animals, administration of 5% dextrose (200 kcal/L) cannot be relied upon to maintain daily caloric needs. In cases in which sepsis is a concern or if hypoglycemia from another cause is present, you can add 100 mL of 50% dextrose (i.e., 50 g) to 1 L of lactated Ringer's solution to create a solution that has 5% dextrose in it. In this situation you are using the dextrose to combat suspected or documented hypoglycemia.
The principle here is that the rate of fluid administration (i.e., how much volume to give over a specific time interval) is dictated by the magnitude and rapidity of the fluid loss. The more severe and rapid the fluid loss, the more rapidly fluids should be administered. In patients with stable chronic disease, use the longest period you have available to you (e.g., 12 or 24 hours). Merely add the dehydration deficit, maintenance needs and estimated ongoing losses and give this amount over this longest period of time you have available.
For example, if you have a vomiting, 10% dehydrated, 10 kg dog you believe to be stable and you are only going to be at the hospital for 12 hours, you may choose to add 1 L for dehydration, 600 mL for maintenance (assuming maintenance to be 60 mL/kg/day) and 500 mL as an estimate of ongoing losses as the dog continues to vomit. You would then give 2,100 mL divided by 12 hours or 175 mL/hr. This infusion rate is 17.5 mL/kg/hr.
If the patient is showing signs of circulating volume compromise (e.g., tachycardia, slow capillary refill time and weak pulses), you may want to give part of the calculated daily needs over a shorter period of time. For example you may choose to give the dehydration deficit of 1 L over the first 4 hours (an infusion rate of 25 mL/kg/hr) and then decrease the infusion rate to what would be required for the maintenance and ongoing losses over the remaining time period (i.e., 1,100 mL over the remaining 8 hours or 13.75 mL/kg/hr).
There isn't a simple answer to how fast you can give fluids; it depends on the circumstances. In shock, remember you can give intravenous fluids at a maximal rate of one blood volume per hour, which corresponds to 80–90 mL/kg/hr in the dog and 50–55 mL/kg/hr in the cat if cardiac function and urine output are normal.
The different products that are available can be really confusing! The table below may help clarify the differences. Note especially the differences in pH and base content as well as the source of the base:
| Lactated Ringer's | Plasmalyte R | Plasmalyte A | Plasmalyte 148 | Normosol R | Dog plasma | |
| Sodium (mEq/L) | 130 | 140 | 140 | 140 | 140 | 145 |
| Potassium (mEq/L) | 4 | 10 | 5 | 5 | 5 | 5 |
| Chloride (mEq/L) | 109 | 103 | 98 | 98 | 98 | 105 |
| Calcium (mEq/L) | 3 | 5 | 0 | 0 | 0 | 5 |
| Magnesium (mEq/L) | 0 | 3 | 3 | 3 | 3 | 3 |
| Base (mEq/L) | 28L |
47A 8L |
27A 23G |
27A 23G |
27A 23G |
24B |
| Osmolality (mOsm/kg) | 272 | 312 | 294 | 294 | 294 | 300 |
| Calories (lcal/L) | 9 | 11 | 21 | 21 | 21 | NA |
| pH | 6.5 | 5.5 | 7.4 | 5.5 | 6.6 | 7.4 |
Note that the base content of all of these crystalloids (except lactated Ringer's solution) is about twice the normal bicarbonate concentration of plasma. Note also, with the exception of its potassium and base content, Plasmalyte R is a pretty close match for plasma. Overall, however, lactated Ringer's solution is probably the best match because its base content is closer to that of plasma.
Lactated Ringer's solution at a rate of 10 mL/kg/hr often is recommended for this purpose. If you wish to read more details about this recommendation, please consult Peter Pascoe's excellent chapter entitled, "Perioperative management of fluid therapy" in S.P. DiBartola: Fluid, Electrolyte, and Acid Base Disorders in Small Animal Practice, 3rd edition, Elsevier, St. Louis, 2006; pp. 406–408.
First, it is entirely appropriate and safe to administer fluids at room temperature to small animals. If you feel it necessary, however, you can heat crystalloid fluids in a microwave as long as you carefully determine the appropriate settings on your particular microwave to heat fluid to no higher than body temperature and carefully invert the bag several times to completely mix the fluid and avoid any pockets of excessive heat within the fluid in the bag.
Administering lactate as a salt (the form present in lactated Ringer's solution) cannot directly cause lactic acidosis. Rather, the ability of the hypoxic liver to metabolize lactate is sometimes questioned. In most situations, administering lactated Ringer's solution probably is beneficial because any tendency toward lactate accumulation is likely to be offset by improved hepatic perfusion and oxygen delivery as a result of extracellular fluid volume expansion.
Potassium can be used in concentrations up to 30 to 35 mEq/L in crystalloid fluids that are intended for subcutaneous use. When supplementing crystalloid fluids with KCl for intravenous use, we traditionally have followed the "sliding" scale shown below that was developed by Dr. Richard Scott at the Animal Medical Center in the 1970s. Intravenous potassium supplementation should NOT exceed 0.5 mEq/kg/hr.
Guidelines for routine intravenous supplementation of potassium*
| Serum potassium concentration (mEq/L) | mEq KCl to add to 250 mL fluid | mEq KCl to add to 1 liter fluid | Maximal fluid infusion rate (mL/kg/hr) |
| <2.0) | 20 | 80 | 6 |
| 2.1–2.5 | 15 | 60 | 8 |
| 2.6–3.0 | 10 | 40 | 12 |
| 3.1–3.5 | 7 | 28 | 18 |
| 3.6–5.0 | 5 | 20 | 25 |
*In dogs and cats
Maintenance solutions contain less sodium and more potassium than replacement fluids. Many maintenance solutions contain approximately 50 mEq sodium per liter.
Consider a 10 kg dog with 6 L of total body water. Four liters is intracellular fluid with a sodium concentration of 10 mEq/L (total of 40 mEq) and 2 liters is extracellular fluid with a sodium concentration of 140 mEq/L (total of 280 mEq). So, the average sodium concentration of the total body water is approximately 320 mEq divided by 6 liters or 53 mEq/L. Thus, maintenance solutions provide a sodium concentration approximating the average sodium concentration of total body water. However, this same approach can't be applied to potassium because excessively rapid intravenous administration of solutions containing large amounts of potassium can be lethal (see question above—the maximum safe intravenous infusion rate for potassium is 0.5 mEq/kg/hr). This is why most maintenance solutions do not contain more than 15 mEq/L of potassium.
Note that these maintenance solutions typically are hypotonic. For example, 0.45% NaCl has an osmolality of 154 mOsm/kg. A solution of 0.45% NaCl in 2.5% dextrose has an osmolality of 280 mOsm/kg, but the dextrose is metabolized by the body rendering this solution effectively hypotonic.
Use colloids when you want to keep as much of the administered fluid in the vessels as possible (remember that crystalloids rapidly—within 15 minutes—distribute to the interstitial space and over several hours distribute to total body water). A colloid is a large molecular weight substance that will stay in the circulating space longer and hence may be indicated in shock patients that require rapid and effective support of their circulating volume.
Another situation where you may want to use a colloid is with a patient with low oncotic pressure due to hypoalbuminemia. The colloid will provide oncotic support in such patients and help hold fluid and electrolytes in the vascular space. Both dextrans and hetastarch typically are supplied in 0.9% NaCl solutions. More information on colloids is found in Chapters 17 and 27 of S.P. DiBartola: Fluid, Electrolyte, and Acid Base Disorders in Small Animal Practice, 3rd edition, Elsevier, St. Louis, 2006.
Yes! If the animal is normal, however, the body is very forgiving of receiving an excess volume of fluid. As long as cardiac pump function and urine output are normal, you are unlikely to experience problems. Many patients probably have benefited from getting a little more fluid than they needed, as opposed to not enough fluid. You can, however, quickly get into trouble and cause overhydration in patients with heart failure and those with pathologic oliguria (e.g., oligoanuric acute renal failure patients).
Acid-Based Disorders
The questions and answers that follow are in response to questions received from the IDEXX-sponsored Webinar:
Advanced Fluid Therapy-An Interactive Case Review of Acid-Base Disorders, presented by Dr. Stephen DiBartola
The short answer is yes. Arterial samples are ideal because oxygenation of blood can be evaluated (important for patients with pulmonary and cardiovascular disease), and the sample is not affected by stasis of blood and local tissue metabolism. Much valuable information, however, can be obtained from careful review and interpretation of venous blood gases using the principles we discussed in the Webinar. Due to peripheral tissue metabolism, venous blood will have a higher PCO2 and lower pH than arterial blood. Consider, for example, the following data derived from normal unanesthetized dogs that appeared in the Journal of Veterinary Internal Medicine (2001;5[5]:294–298).
| Parameter | Arterial blood | Jugular venous blood |
| pH | 7.395 ± 0.028 | 7.352 ± 0.023 |
| PCO2 (mm Hg) | 36.8 ± 2.7 | 42.1 ± 4.4 |
| Bicarbonate (mEq/L) | 21.4 ± 1.6 | 22.1 ± 2.0 |
The short answer is yes. The literature contains many concepts for assessing acid-base balance. Some, such as standard bicarbonate and base excess, are predicated on the notion (which is an oversimplification) that the buffering capacity of whole blood reflects the whole animal's buffering capacity. Others, such as the strong ion difference, are conceptually sound but mathematically sophisticated and too cumbersome for general everyday use. If you have a good understanding of the principles of acid-base chemistry and physiology, "yes," pH, PCO2 and bicarbonate are sufficient as discussed in the Webinar.
Total CO2 is determined by adding a strong acid to plasma or serum and measuring the amount of CO2 released from the reaction: H+ + HCO3– → H2CO2 → CO2 + H2O.
The term total CO2 refers to the fact that this method includes both the dissolved CO2 and bicarbonate present in the sample. As a result, total CO2 determined on an anaerobically handled sample is approximately 1 or 2 mEq/L higher than the bicarbonate concentration because dissolved CO2 (in mEq/L) = PCO2 (in mm Hg) × 0.03 (the solubility coefficient for CO2). However, when the sample is handled aerobically (as typically is the case for blood samples sent to a laboratory), the dissolved CO2 is released to the atmosphere, and the value obtained essentially is equal to the bicarbonate concentration of the sample. That's a long way of saying that total CO2 on a routinely handled biochemical profile is the same thing as the bicarbonate concentration.
The value of that information will depend on the clinician's understanding of acid-base balance. For example, what does a low bicarbonate concentration mean? Most often, it will signify the presence of metabolic acidosis, but (although much less likely) it also could reflect a decrease in the bicarbonate concentration as compensation for a primary respiratory alkalosis. Blood–gas analysis would be necessary to answer this question definitively if the answer is not already clear from the patient's clinical presentation.
As another example, what does a high bicarbonate concentration mean? Most often, it will signify the presence of metabolic alkalosis, but it also could reflect an increase in the bicarbonate concentration as compensation for a primary respiratory acidosis. Again, blood-gas analysis would be necessary to answer this question definitively if the answer is not already clear from the patient's clinical presentation. Thus, a good understanding of acid-base principles will help you interpret the meaning of the total CO2 on a biochemical profile.
This depends on whether or not the patient is stable. The more unstable the patient, the more frequently acid-base parameters should be monitored. In critical patients, monitoring could be necessary every few hours. In critically ill patients judged to be in need of sodium bicarbonate therapy (based on presence of a severely low pH such as 7.0–7.1), one common practice is to administer a low dose of bicarbonate (e.g., 1–2 mEq/kg) slowly by the intravenous route and then reevaluate blood gases in a few hours.
This depends upon the clinical condition and the clinician's preference. In most instances, the decision to administer bicarbonate is made in a critically ill patient (e.g., blood pH ≤ 7.1), and often a low dose of bicarbonate (1–2 mEq/kg) is administered slowly by the intravenous route. Alkalinizing crystalloid fluids (e.g., lactated Ringer's solution) are often administered also. When blood gases are checked several hours later, it is not unusual to observe that the acidosis is well on its way to resolution and nothing more needs to be done in terms of bicarbonate administration.
In the unusual situation where severe metabolic acidosis and extremely low bicarbonate concentration are refractory to initial treatment, the clinician may elect to start a constant rate infusion of bicarbonate, but important questions remain. For example, what volume of distribution (Vd) should be used in the calculation of the amount of bicarbonate to give? The general formula is mEq bicarbonate = Vd × body weight (kg) × bicarbonate deficit (mEq/L). You will see values of 0.2 (extracellular space) to 0.6 (total body water) used for Vd in this equation, and (experimentally at least) the Vd for bicarbonate in dogs with severe chronic metabolic acidosis can even exceed 0.6. If you are going to use this kind of equation, you should probably pick a low value such as 0.2 for Vd until you see how the patient responds.
Another question is how to calculate the "bicarbonate deficit." Do you simply subtract the patient's bicarbonate concentration from the normal value of 21 mEq/L and plug that result into the equation above as the "bicarbonate deficit?" You probably shouldn't, because your goal is not to return the patient's bicarbonate concentration to completely normal but just enough to increase the pH out of the danger zone (i.e., to above 7.2). The amount of bicarbonate needed to do so likely will be less than the amount calculated from the equation.
From these considerations, you can see that it is not simply a matter of plugging numbers into an equation. Consequently, it's often preferable to give repeated small doses of sodium bicarbonate until the patient's blood pH stabilizes above 7.2. This approach requires serial reevaluation of the patient's blood gases and adjustments to treatment based on the results obtained.
Opinions differ about the value of base excess in the evaluation of acid-base status. Base excess is defined as the amount of strong acid or base required to titrate 1 liter of blood to pH 7.40 at 37°C while PCO2 is held constant at 40 mm Hg. Base excess is changed only by nonvolatile (or fixed) acid and thus is considered to reflect the magnitude of metabolic acid-base disturbances. A negative value indicates metabolic acidosis and a positive value metabolic alkalosis (normal base excess is approximately -3 to +3 mEq/L). People often use changes in base excess to determine the presence of a metabolic acid-base disorder. However, the observed change in the patient's bicarbonate may reflect a normal adaptive renal response to the presence of a primary respiratory acid-base disorder. If the clinician understands the implications of the Henderson-Hasselbalch equation and follows the guidelines for interpreting blood-gas data that we discussed during the Webinar, there is no real need to bring base excess into the discussion.
The "anion gap" simply is the difference between the "commonly measured" cations (sodium and potassium) and the "commonly measured" anions (chloride and bicarbonate). In reality, the law of electroneutrality must be satisfied and there is no actual anion gap. It's just that there are more unmeasured anions (i.e., negative charges on proteins, phosphate, sulfate and lactate as well as other organic anions) than unmeasured cations (i.e., only calcium and magnesium).
In some types of metabolic acidosis, fixed acids decrease serum bicarbonate concentration, and the anions of these acids accumulate as unmeasured anions. Examples include:
- Diabetic ketoacidosis, where organic ketoanions accumulate
- Ethylene glycol poisoning, where organic anion metabolites of ethylene glycol accumulate
- Renal failure, where phosphate accumulates
- Lactic acidosis, where the organic anion lactate accumulates (pictured in the diagram above on the left)
This type of acidosis is called a "high anion gap" or "normochloremic" acidosis because the unmeasured anion increases in proportion to the decrease in bicarbonate and serum chloride concentration is unchanged. This is the most common type of metabolic acidosis encountered in veterinary medicine.
The other type of acidosis, pictured in the diagram above on the right, is called a "normal anion gap" or "hyperchloremic" acidosis, because there is no accumulated unmeasured anion and serum chloride concentration increases in proportion to the decrease in serum bicarbonate. This type of acidosis is somewhat less common in veterinary medicine and is exemplified by small-intestinal diarrhea. In this situation, bicarbonate-rich fluid is lost in the diarrhea, the animal becomes dehydrated and the kidneys increase their absorption of sodium and water. Because of the lower serum bicarbonate concentration, the filtered load of bicarbonate is decreased and the kidneys must reabsorb more of the sodium with chloride, leading to hyperchloremia. Other causes of hyperchloremic acidosis, such as renal tubular acidosis, are rare.
This hits upon a very important issue—namely that we determine the patient's expected compensatory response by doing calculations based on "average" normal values, whereas in reality, we must deal with normal reference ranges from laboratories and we don't really know what the actual "normal" value is for any individual patient. In the case of pH, we typically use 7.38 as an average "normal" value for arterial samples. As a consequence of this uncertainty, we conclude that an acid-base disturbance is a simple disorder as long as the observed compensatory PCO2 (in the case of metabolic disorders) or compensatory bicarbonate concentration (in the case of respiratory disorders) is within 2–3 mm Hg (for PCO2) or 2–3 mEq/L (for bicarbonate) of the calculated value. Only when the observed values are more than 2–3 mm Hg or 2–3 mEq/L away from the calculated values do we consider the possibility of a mixed disorder, and it is always important to ask yourself if your conclusions are warranted by the patient's clinical presentation.
Educational Partner
IDEXX is an Educational Partner of the American Association of Equine Practitioners

Quick Links
*These parameters are calculated from parameters measured by the VetStat analyzer.